| Drug Name | TT-173 |
| Description |
TT-173 is the first human tissue factor (TF)- based hemostat, compared to the commonly used topical thrombin as surgical hemostats. It is produced by expressing recombinant human TF in yeast, purifying cell membranes, and forming the microvesicles. Using this approach, a pharmaceutical grade hemostat can be obtained easily and cost effectively. TT-173 is presented as lyophilized powder in glass vials intended to be reconstituted just before application. The resulting hemostatic solution can be sprayed over the bleeding surfaces with a manual syringe coupled to a nozzle diffuser or, alternatively, can be applied on a gelatin sponge or similar support over the bleeding area. This therapeutic compound has been granted the Orphan Drug Designation for hemophilia and von Willebrand disease. The study of TT-173 is currently in phase II/III clinical trial for total knee arthroplasty (TKA), after completing the successful phase I in dental extraction and phase II in skin grafting. |
| Active Ingredient | Tissue factor |
| Drug Modality | Recombinant protein |
| Indication | Surgical bleeding and trauma |
| Product Category | Hemostatic |
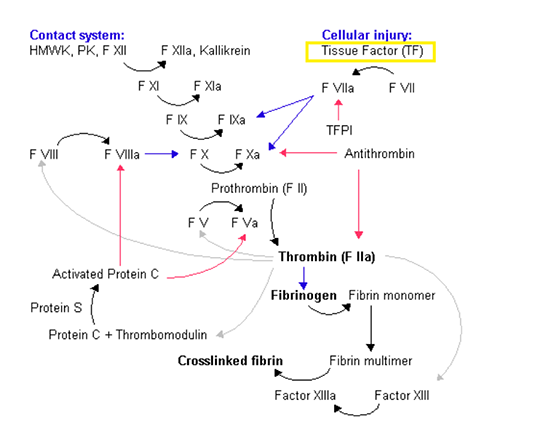
| Mechanism of Action | Tissue factor, the active ingredient of TT-173, induces the activation of extrinsic pathway of the coagulation cascade to reduce blood loss. |
| Status | Phase II/III |
| Patent | Filed |
Protheragen Inc. is actively seeking partnership to further develop TT-173. Potential collaboration can be strategic alliance, licensing, or other marketing agreement.
We look forward to hearing from you.
| Introduction |
This gene encodes coagulation factor III, which is a cell surface glycoprotein. This factor enables cells to initiate the blood coagulation cascades, and functions as the high-affinity receptor for the coagulation factor VII. The resulting complex provides a catalytic event that is responsible for initiation of the coagulation protease cascades by specific limited proteolysis. Unlike other cofactors of these protease cascades, which circulate as nonfunctional precursors, this factor is a potent initiator that is fully functional when expressed on cell surfaces. There are 3 distinct domains of this factor: extracellular, transmembrane, and cytoplasmic. This protein is the only one in the coagulation pathway for which a congenital deficiency has not been described. Alternate splicing results in multiple transcript variants. |
| Approved Name | Coagulation factor III, tissue factor |
| Official Symbol | F3 |
| Gene Type | Protein coding |
| Synonyms | TF; TFA; CD142 |
| Ensembl | ENSG00000117525 |
| Gene ID | 2152 |
| mRNA Refseq | NM_001993 |
| Protein Refseq | NP_001171567 |
| OMIM | 134390 |
| UniProt ID | P13726 |
| Chromosome Location | 1p21.3 |
Clinical Resources
| Gene Function | TF expression from adventitial fibroblasts and vascular smooth muscle cells surrounding blood vessels provides a hemostatic barrier that activates coagulation when vascular integrity is disrupted. TF is also expressed by cardiac muscle but not skeletal muscle. The coagulation protease cascades are composed of the extrinsic (TF/FVIIa) and intrinsic (FVIIIa/FIXa) pathways, which together maintain hemostasis. |
| Pathway | Coagulation cascade |
| Major Conditions | Eye disorders; cancer |
TT-173 is the first recombinant human Tissue Factor-based topical hemostat for use in surgical bleeding. Developed as a lyophilized, it is highly stable even at room temperature. Its use can be flowable gelatin matrix, presentation in powder, absorbable dressing, and sealant.
Bleeding is a potential complication of any surgical procedure, representing a major challenge for surgeons and anesthetists. The larger and more complex the surgery, the greater the potential for unexpected severe bleeding. Although the mortality rate is low for most surgical procedures, that is, less than 0.1% for most routine surgery, the percentage may be greatly increased when severe bleeding occurs during the operative procedure. Severe, unexpected, and uncontrollable bleeding during the operation can raise the mortality rate from 1% to 20%.
The most common cause of significant intraoperative bleeding is inadequate surgical hemostasis. Moreover, it has been demonstrated that surgical technique per se affects the rate of postoperative hemorrhage. However, in some cases either acquired or congenital coagulopathies may at least favor, if not directly, cause surgical bleeding. Whatever the cause, uncontrolled bleeding can lead to a combination of hemodilution, hypothermia, consumption of clotting factors, and acidosis, which in turn exert negative influences over the clotting process to further exacerbate the problem in a vicious circle.
When surgical hemostasis is inadequate or impractical, topical hemostatic agents are used. Most routine, elective surgeries are performed on patients with normal hemostasis and minimal blood loss. There are two main types of local hemostats: one is mechanical hemostats that use passive substrates to promote hemostasis; the other is bioactive hemostatic agents, which enhance coagulation at the site of bleeding.
Tissue factor (TF) is a transmembrane glycoprotein that induces the activation of extrinsic pathway of the coagulation cascade. The use of TF as a hemostat has been postulated in the past, but their development as hemostatic treatment has been limited by the fact that purified TF presents a very limited biological activity and must be incorporated into a lipid membrane to adopt and adequate folding.
TT-173 is the first recombinant human tissue factor-based, not thrombin-based, hemostatic agent, using yeast-derived microvesicles integrating a modified form of recombinant human TF in the membrane. As a fully recombinant compound, TT-173 does not present associated risk of infectious agent transmission. In contrast to thrombin, TT-173 acts at the first step of coagulation cascade activating all the coagulation factors, stopping the hemorrhage with better mechanical properties and leading to a higher hemostatic efficacy compared to thrombin.
Vojnosanit Pregl 2017; 74(10): 974–981
TT-173 is currently in phase II/III study in total knee arthroplasty (TKA), after successfully completing the phase I in dental extraction and phase II in skin grafting. Short term strategic objectives (18 months) are to complete the final clinical trials and drug registration with the European Medicines Agency (EMA) and the US FDA.
