| Drug Name | Anti-CD137-6051 |
| Description |
Anti-CD137-6051 is a humanized IgG monoclonal antibody targeting CD137 (also known as TNFRSF9 and 4-1BB) with an engineered Fc capable of selectively binding to the Fcγ receptor IIB (FcγRIIB). Anti-CD137-6051 acts as a conditional CD137 agonist to result in optimal immune activation in tumor microenvironment. Anti-CD137-6051 is in clinical development as monotherapy and in combination with anti-PD-1 antibody for the treatment of advanced or metastatic malignancies. |
| Target | CD137 |
| Drug Modality | Monoclonal antibody |
| Indication | Advanced or metastatic malignancies |
| Product Category | Cancer immunotherapy |
| Mechanism of Action | FcγRIIB mediated CD137 activation |
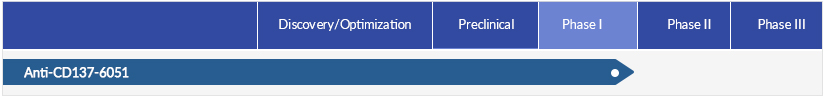
| Status | Phase 1 |
| Patent | Granted |
Protheragen Inc. is actively seeking partnership for Anti-CD137-6051. Potential collaboration can be strategic alliance, licensing, or marketing agreement.
We look forward to hearing from you.
| Introduction | CD137 is a member of the tumor necrosis factor receptor superfamily, which helps regulate the activation of many immune cells, including CD4+ T cells, CD8+ T cells, dendritic cells, and natural killer cells. CD137 is considered as a promising target for enhancing antitumor immune responses. |
| Approved Name | TNF receptor superfamily member 9 |
| Official Symbol | TNFRSF9 |
| Gene Type | Protein coding |
| Synonyms | 4-1BB |
| Ensembl | ENSG00000049249 |
| Gene ID | 3604 |
| mRNA Refseq | NM_001561 |
| Protein Refseq | NP 001552 |
| OMIM | 602250 |
| UniProt ID | Q07011 |
| Chromosome Location | 1p36.23 |
| Gene Function | Human CD137 gene encodes a 255-amino acid protein with two potential N-linked glycosylation sites that contributes to the clonal expansion, survival, and development of T cells. CD137 can also induce peripheral monocyte proliferation, enhance T cell apoptosis induced by TCR/CD3 triggered activation, and regulate CD28 co-stimulation to promote Th1 cell responses. |
| Major Conditions | Cancers |
Based on a proprietary monoclonal antibody functional platform, Anti-CD137-6051 is developed with tumor-localized immunostimulatory activities by balancing multiple functions of antibodies. FcγRIIB is expressed on immune cells enriched in the tumor microenvironment, including B cells, monocytes, and NK cells. Anti-CD137-6051 is a weak agonistic antibody which required FcγRIIB-mediated cross-linking for optimal agonistic activity.
According to the WHO Global Cancer Observatory (GLOBOCAN), cancer was a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020. Overall, lung, colon and rectum, and liver were the three most deadly cancers. 18.09 million new cases of cancer had been diagnosed in 2020 in which breast, lung, colon and rectum, prostate, and skin being the five most frequent. Advanced cancer cannot be cured with treatment. Metastatic cancers are generally considered to be advanced if it cannot be cured or controlled with treatment. However, some types of advanced cancers can be controlled with treatment over a long period of time and are considered as chronic diseases. Proper treatment regimen can shrink the cancer, slow its growth, and prolong the life of patients.
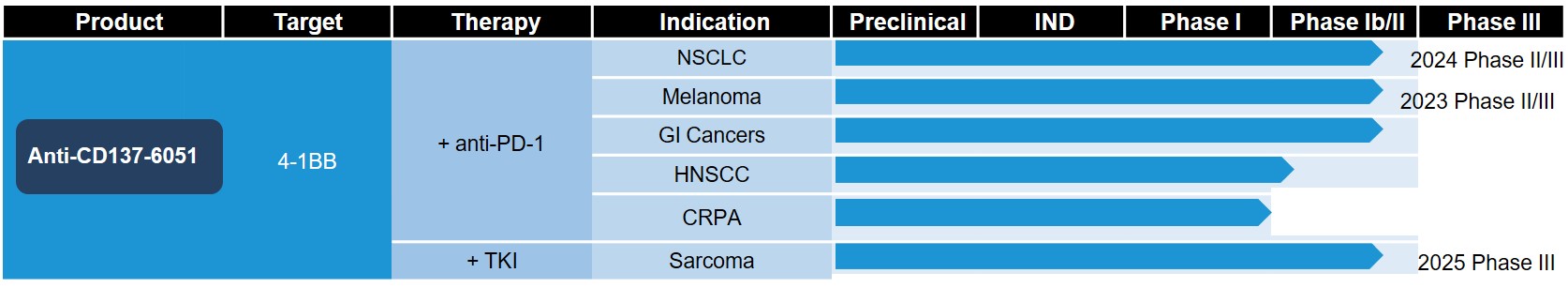
Anti-CD137-6051 as monotherapy or in combination with anti-PD-1 antibody or vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR-TKI) is in clinical development for the treatment of adult patients with advanced malignancies. Preliminary antitumor activity in patients was observed. Anti-CD137-6051 combined with anti-PD-1 antibody therapy induced rapid antitumor responses in patients with immune-cold tumors or relapsed from prior immunotherapies.
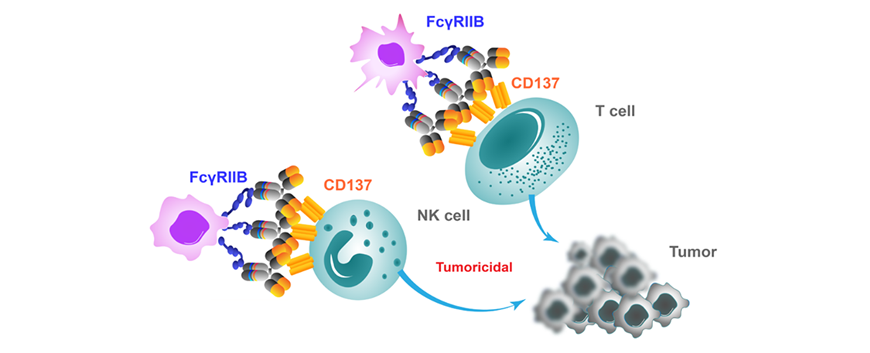
Immunotherapy has achieved considerable success in the field of cancer treatment. T cell activation is a key process in the fight against cancer, in which both costimulatory and coinhibitory signals play a critical role. CD137 is a co-stimulatory immune checkpoint molecule that regulates the activity of a variety of immune cells. It strongly activates CD8+ T cells, induces cytokine release, and enhances cytotoxic T lymphocytes activity.
Anti-CD137-6051 is a weak agonistic antibody that requires FcγRIIB-mediated cross-linking for optimal agonistic activity. The co-stimulatory effect of Anti-CD137-6051 is significantly enhanced in the presence of FcγRIIB. Anti-CD137-6051 shows comparable T cell costimulatory ability to a urelumab analogue, and is superior to a utomilumab analogue in the presence of FcγRIIB-expressing cells. Importantly, Anti-CD137-6051 does not induce hepatotoxicity while maintaining potent antitumor activity. Anti-CD137-6051 with outstanding safety profile has the potential to be used as monotherapy or in combination with other anticancer drugs to treat cancer.
The phase I trial of Anti-CD137-6051 monotherapy and the phase Ia trial of Anti-CD137-6051 and anti-PD-1 antibody combination therapy have been completed in adult patients with advanced malignancies. In clinical trials, Anti-CD137-6051 showed good safety and antitumor activity.