| Drug Name | Polyoxygenated cyclohexene compound 4 |
| Description |
Sodium channels (Nav) are closely associated with nervous system activities. Changes in the biology of these channels can be associated with various types of channelopathy. In recent years, many studies have shown that the Nav1.7 located at the endings of pain-sensing nerves is closely related to inflammatory pain and neuropathic pain. The Nav1.7 inhibitors developed by our partners are a class of polyoxygenated cyclohexenes. The efficacy tests and in vivo experiments show that polyoxygenated cyclohexene 4 significantly decreased Nav1.7 activity and had significant analgesic effects on neuropathic pain, inflammatory pain, and nociceptive pain. |
| Target | Nav1.7 (sodium voltage-gated channel alpha subunit 9) |
| Drug Modality | Small molecule chemical drug |
| Indication | Pain |
| Product Category | Analgesic drug |
| Mechanism of Action | The compound prevents and treats pain by inhibiting activity of Nav1.7. |
| Status | Preclinical |
| Patent | Granted |
Protheragen Inc. is actively seeking partnership to further develop polyoxygenated cyclohexene compound 4. Potential collaboration can be strategic alliance, licensing, or marketing agreement.
We look forward to hearing from you.
| Introduction | Sodium voltage-gated channel alpha subunit 9 (Nav1.7) is a voltage-gated sodium channel that is enriched in nociceptive and sympathetic neurons of the peripheral nervous system. It is also expressed in subcortical structures of brain. Nav1.7 mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na ions may pass in accordance with their electrochemical gradient. Nav1.7 plays a role in pain mechanisms, especially in the development of inflammatory pain. |
| Approved Name | Sodium voltage-gated channel alpha subunit 9 |
| Official Symbol | SCN9A |
| Gene Type | Protein coding |
| Synonyms | Nav1.7; PN1; NE-NA; NENA; ETHA |
| Ensembl | ENSG00000169432 |
| Gene ID | 6335 |
| mRNA Refseq | NM_002977; NM_001365536 |
| Protein Refseq | NP_002968; NP_001352465 |
| OMIM | 603415 |
| UniProt ID | Q15858 |
| Chromosome Location | 2q24.3 |
| Gene Function | This gene encodes a voltage-gated sodium channel which plays a significant role in nociception signaling. Mutations in this gene have been associated with primary erythermalgia, channelopathy-associated insensitivity to pain, and paroxysmal extreme pain disorder. |
| Pathway | Nav1.7 controls the voltage-dependent sodium permeability of the channel on excitable membranes. |
| Major Conditions | Pain, respiratory disorders, psychiatric disorders, cancer, etc. |
Polyoxygenated cyclohexene compounds are a class of potential therapies that can be used to prevent or treat pain. Moreover, polyoxygenated cyclohexene compound 4 obtained through screening has the characteristics of low toxicity and high bioavailability.
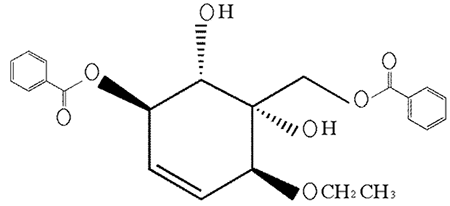
Figure. The formula of polyoxygenated cyclohexene compound 4
Pain is a highly complex, heterogeneous and dynamic process that involves multiple interrelated neurotransmitter and neuromodulator systems in the spinal cord, ascending and descending spinal pathways and supraspinal sites. As a vital physiological function, pain constitutes the body’s mechanism of self-preservation; it serves as a warning to indicate harm or impending danger to body tissues and the need to avoid injury and/or take care of oneself. The same sensation, however, has the potential to evolve into a chronic, debilitating disease under certain pathological conditions such as inflammation, cancer, viral infection, diabetes, etc.
According to the National Hospital Ambulatory Medical Care Survey, approximately 45.4% of emergency department visits in the U.S. from 2000-2010 involved a primary symptom or diagnosis of pain. Arthritis, headache disorders including migraine, fibromyalgia and chronic low back pain are among the most common sources of chronic pain. Anywhere from 70-90% of advanced cancer patients experience significant pain as a result of their condition, and more than half of hospitalized patients experience moderate to severe pain during their last days of life.
Pain has a considerable economic impact at the individual and societal levels. The economic burden associated with persistent pain, which includes healthcare utilization, quality of life and impact on productivity, absenteeism and risk of leaving the labor market, is comparatively greater than most other health conditions.
Currently, the main drugs for the treatment of pain are non-steroidal anti-inflammatory drugs and opioid analgesics. However, the analgesic effect of non-steroidal drugs is insufficient, and they produce digestive system-related side effects. Opioid analgesics can easily lead to side effects such as nausea, vomiting, constipation, and dependence, and are often not effective on neuropathic pain. Thus, there is a great need to develop new drugs with good analgesic effect and fewer side effects.
Nav1.7 is widely regarded as a crucially important ‘pain channel’ that plays a key role in human pain. Nav1.7 is expressed in peripheral somatic and visceral sensory neurons within the dorsal root ganglia (DRG), including most functionally identified nociceptors, and in sympathetic ganglion neurons, myenteric neurons, and olfactory sensory neurons. Because of its voltage-dependence and slow rate of closed-state inactivation, Nav1.7 is activated in response to slow, low-amplitude depolarizations below the threshold for action potential rise, thereby amplifying small stimuli. Nav1.7 thus sets the gain on cells in which it is present. Additional evidence is provided by the demonstration that selective blockade of Nav1.7 increases firing threshold in both rodent and human DRG sensory neurons, and reduces neurotransmitter release from peripheral and central terminals. Global or conditional knockout of Nav1.7 have been shown to ameliorate pain and increases pain threshold.
The Nav1.7 inhibitors developed by our partners are a class of polyoxygenated cyclohexene. Polyoxygenated cyclohexene compound 4 was optimized by various experiments. To test its potential inhibition on Nav1.7, a number of comparative in vivo experiments have been carried out. The efficacy tests and in vivo experiments show that polyoxygenated cyclohexene compound 4 significantly decreased Nav1.7 activity and have significant analgesic effects on neuropathic pain, inflammatory pain, and nociceptive pain.
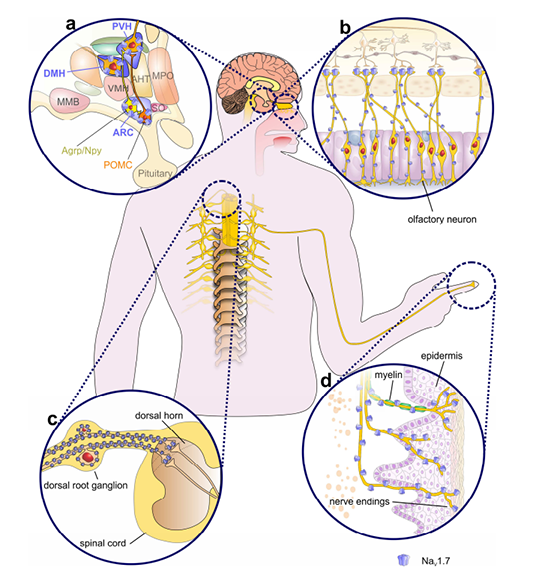
Figure. Expression profile of NaV1.7
Pharmacology and Therapeutics (April, 2017), 172:73-100
In order to test the efficacy of the polyoxygenated cyclohexene compound 4 (compound 4) in preventing and treating pain, several trials of pain threshold testing have been completed:
● Pain hypersensitivity test in chronic sciatic nerve compression injury model;
● Abdominal constriction test induced by glacial acetic acid;
● Hot water tail flick test.
