| Drug Name | PMB-104 |
| Description |
PMB-104 is a biobetter antibody of a marketed anti-IgE antibody for the treatment of allergic diseases. Compared with the reference marketed drug, PMB-104 showed higher affinity and greater efficacy in vitro and in vivo, and a longer half-life in healthy volunteers of clinical trial. PMB-104 is developed as a more convenient injectable formulation for clinical administration. |
| Target | Immunoglobulin E (IgE) |
| Drug Modality | Optimized antibody |
| Indication | Allergic asthma and other allergic diseases |
| Product Category | Immunotherapy |
| Mechanism of Action | PMB-104 binds to the constant region (Fc) of free IgE and prevents free IgE from binding to IgE receptors, thereby inhibiting effector cell responses to allergens. |
| Status | Phase I |
| Patent | Granted |
Protheragen Inc. is actively seeking partnership for PMB-104. Potential collaboration can be strategic alliance, licensing, or marketing agreement.
We look forward to hearing from you.
Immunoglobulin E (IgE) is is one of five classes of immunoglobulins (IgM, IgG, IgD, IgA, IgE) and is found only in mammals. The IgE monomer consists of two heavy chains (ε chains) and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). Thus, IgE is able to bind a total of two antigens, which occurs in variable regions of the light and heavy chains that produce unique antigen-specific binding sites.
IgE plays a key role in in allergic and atopic disorders such as allergic rhinitis, asthma, and atopic dermatitis. Initial exposure to antigen or allergen is taken up and processed by dendritic cells or macrophages, which present the antigen to T cells. These T cells are induced to differentiate into Th2-helper T cells capable of presenting antigens to B cells. B cells then produce IgE antibodies that bind to the presented antigen at the antigen-binding site the antibody. Once this initial sensitization to the antigen has occurred, more immunologic events can ensure a more robust IgE response. Fc-ε-RII (CD23) on intestinal and airway epithelial cells can transport antigen-IgE complexes across the epithelium to bind to Fc-ε-RI receptors on mast cells, macrophages, and other immune cells, thereby promoting inflammatory cytokines and more local IgE production. Repeated exposure to allergens leads to crosslinking of the Fc-ε-RI-bound IgE in mast cells and basophils, inducing cell degranulation and release of histamine, trypsinoid, cysteine-leukotriene, and platelet-activating factor. These cytokines can cause allergic reactions.
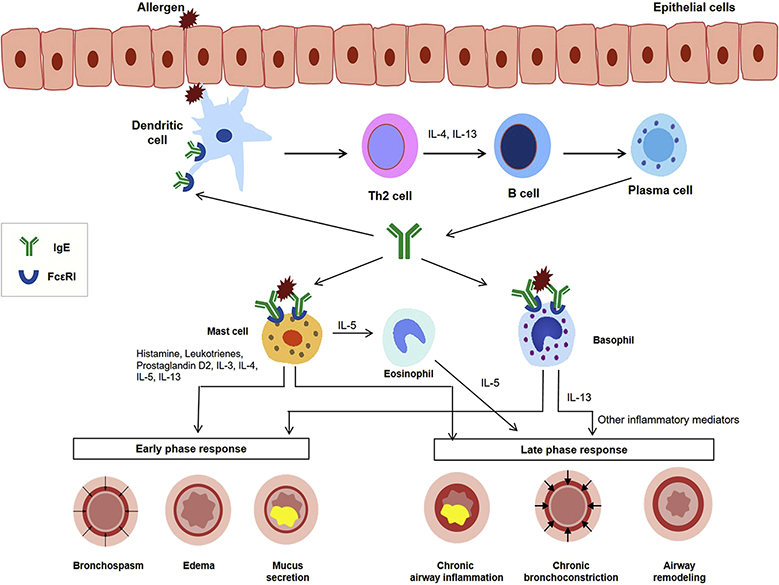 Immunological mechanisms in IgE-mediated allergic diseases.
Immunological mechanisms in IgE-mediated allergic diseases.
(J Allergy Clin Immunol Pract. 2019;7(5):1418-1429.)
The biobetter antibody PMB-104 is developed to overcome the limitations of Omalizumab, such as chemical instability, lower affinity to IgE, and immunogenicity risk. Antibody engineering technology was used to optimize the sequence of the original antibody to reduce the potential immunogenicity risk, and two typical Asp isomerization hotspots in the complementarity determining regions (CDRs) were removed to improve the stability. After screening out the candidate antibodies with higher affinity, crystallizable fragment (Fc) engineering was applied to prolong the serum half-life of antibody.
Allergic asthma is a chronic airway inflammatory disease in which allergen exposure can cause intermittent attacks of breathlessness, airway hyper-reactivity, wheezing, and cough. Allergic asthma is a syndrome caused by a complex interplay between genetic and environmental factors. Over 300 million individuals worldwide suffer from asthma, and in the United States, more than 35 million people are estimated to have this disease, most of them children. The prevalence and impact are particularly rising, and it is estimated that an additional 100 million people could develop asthma by 2025. Allergic asthma is the most common asthma phenotype. It is estimated that up to 80% of childhood asthma and more than 50% of adult asthma cases may have an allergic component. The Third National Health and Nutrition Examination Survey estimated that 56% of asthma cases in the United States were associated with atopy.
There is no cure for allergic asthma, but symptoms can be improved. There are multiple treatments for allergic asthma, including environmental control measures, immunotherapy, glucocorticoids, and biologics. The choice of treatment is mainly based on the severity of the disease and the frequency of symptoms. Although current therapeutics are effective in improving asthma symptoms, their use is still limited. About half of patients with asthma worldwide remain sub-optimally controlled, even when treated.
PMB-104 is humanization antibody that binds to free IgE with high affinity, thereby preventing allergen-specific IgE from attaching to FcεRI on eosinophils, basophils, and mast cells, interrupting the allergic cascade. In addition, the decrease in free IgE levels results in a decrease in the number of FcεRI receptors on mast cells, basophils, and antigen-presenting cells. PMB-104 showed a 3.9-fold increase in affinity with IgE compared to Omalizumab. In the clinical trial, PMB-104 significantly improved lung function and was well tolerated in all dose groups (AE≤ grade 1).
Patent was filed.
