| Drug Name | RNA4TNBC |
| Description |
RNA4TNBC are designed as RNA Nanoparticles (NPs) to deliver microRNA 34a (MIR34A) mimics and anti-MIR21 microRNA with specific tumor targeting and minimal side effects and toxicity to prevent tumor proliferation and migration. MIR34A mimics could control targets proteins involved in cell cycle, differentiation and apoptosis, and antagonize process. Anti-MIR21 microRNA inhibits breast cancer cell proliferation and metastasis. |
| Target | MIR34A; MIR21 |
| Drug Modality | RNA nanoparticle |
| Indication | Triple-negative breast cancer (TNBC) |
| Product Category | Small RNA therapeutic |
| Mechanism of Action | Delivering antagomir against oncogenic miRNA and tumor suppressor miRNA using RNA NPs for TNBC treatment. |
| Status | Preclinical |
| Patent | Granted |
Protheragen Inc. is actively seeking partnership for RNA4TNBC. Potential collaboration can be strategic alliance, licensing, or marketing agreement.
We look forward to hearing from you.
| Introduction | microRNA 34a (MIR34A), a member of the highly conserved MIR34 family, is ubiquitously expressed in normal human tissues. In human genome, MIR34A is encoded on chromosome 1p36.
MIR34A is transcribed as a long hairpin molecule, which is subsequently cleaved by RNase III Drosha to a ~ 70-nucleotide long stem-loop precursor (pre-miRNA). Following nuclear export, the pre-miRNA is further cleaved by the RNase III Dicer into 22-nucleotide long mature strands, which are incorporated into RNA-induced silencing complex. This RNA/protein complex mediates downregulation of target transcripts by mRNA degradation or inhibition of translation. |
| Approved Name | microRNA 34a |
| Official Symbol | MIR34A |
| Gene Type | RNA, micro |
| Synonyms | hsa-MIR34a |
| Ensembl | ENSG00000284357 |
| Gene ID | 407040 |
| mRNA Refseq | NR_029610 |
| OMIM | 611172 |
| Chromosome Location | 1p36.22 |
| Gene Function | MIR34A is a candidate tumor suppressor miRNA based on its frequent deregulation in cancer tissues and its ability to regulate the expression of multiple targets implicated in tumorigenesis and cancer progression, such as MYC, MET, CDK4/6, NOTCH1, BCL2, CD44 and many other molecules. |
| Pathway | MIR34A regulates cell survival, migration, and remodeling via repression of target genes and modulation of downstream signaling pathways. |
| Major Conditions | Cancers |
| Introduction | microRNA21(MIR21) was one of the first mammalian microRNAs identified. Its mature sequence is strongly conserved throughout evolution. The human MIR21 gene is located on plus strand of chromosome 17q23.2 within a coding gene TMEM49. |
| Approved Name | microRNA 21 |
| Official Symbol | MIR21 |
| Gene Type | RNA, micro |
| Synonyms | MIRN21; mir-21 |
| Ensembl | ENSG00000284190 |
| Gene ID | 406991 |
| mRNA Refseq | NR_029493 |
| OMIM | 611020 |
| Chromosome Location | 17q23.1 |
| Gene Function | In cells, MIR21 functions as an anti-apoptotic and pro-survival factor. High expression levels of MIR21 may be an indication for cancer cells and represent a common feature of pathological cell growth or cell stress. In addition, the induction of miR-21 is associated with cellular de-differentiation. These findings led to the hypothesis that relatively low levels of miR-21 may be temporarily and spatially required for differentiation and development, whereas high levels may exert oncogenic effects. |
| Pathway | Inhibiting the expression of phosphatases to limit the activity of signaling pathways such as AKT and MAPK |
| Major Conditions | Cancers |
Using RNA nanotechnology platform and RNA NPs as vectors, RNA4TNBC delivers MIR34A mimics and anti-MIR21 microRNA into cancer cells. The delivery efficiency is enhanced, and target effect and toxicity can be greatly reduced.
Breast cancers occur with high incidence and high mortality rate in the female population worldwide. However, at a microscopic and molecular level, breast cancer is not a homogeneous disease. Triple-negative breast cancer (TNBC) is a subtype that does not express the genes for estrogen receptor (ER), progesterone receptor (PR), and HER2/neu.
Though only 10–15% of primary breast cancers are triple negative, TNBCs account for a disproportionate number of patient deaths, with a 5-year survival rate after diagnosis in 83% TNBC patients, measured in female patients with operable invasive breast cancer by statistics from 2010 to 2015 in the Surveillance, Epidemiology, and End Results (SEER) database.
Because hormone therapy and HER2 drugs are not for women with TNBC, chemotherapy is often used. Available treatment for TNBC is still an unmet need due to the lack of effective therapeutics on the market.
MIR21 is a miRNA that promotes tumor invasion and metastasis and is highly expressed in a variety of tumors. Tumorigenesis, invasion, and metastasis promoted by MIR21 are mainly through inhibiting target genes such as PTEN, PDCD4, TPM1, Maspin, and SPRY2, all of which negatively regulating tumor growth, invasion, and metastasis.
MIR34A is considered a tumor suppressor gene, and its downstream target genes include Cyclin D1, Cyclin E2, CDK4, and CDK6, all important factors regulating the cell cycle. Other downstream target genes of MIR34A include Fra-1, Myc, SIRT1, Notch1, and Bcl-2. MIR34A can improve the responsiveness of breast cancer cells to drugs by regulating Notch1.
Using the RNA Nanoparticle technology, RNA4TNBC can deliver MIR34A mimics and anti-MIR21 microRNA into breast cancer cells with less side effects and low toxicity. By synergistic anticancer action, RNA4TNBC can effectively inhibit tumor growth and metastasis and enhance the sensitivity of tumor cells to chemotherapy.
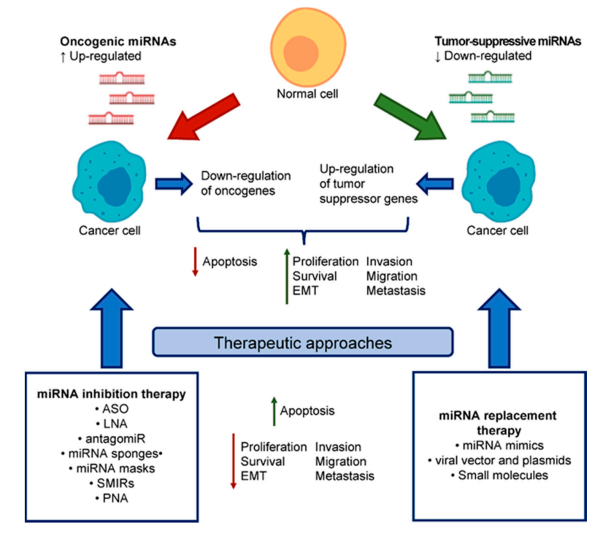
Figure from Int. J. Mol. Sci. 2020, 21, 2805
Most of pharmacodynamics, toxicology, and pharmacokinetics tests in vivo have been completed. The IND application is being prepared.
