| Drug Name | ZM-003 |
| Description |
Epilepsy is one of the most common neurologic diseases affecting approximately 70 million people worldwide. In the pathophysiology of epilepsy, KCNQ2/3 potassium channels play key role for cellular excitability in the brain. Modified from Retigabine (the first approved KCNQ2/3 potassium channel activator drug but withdrawn from market in 2017), ZM-003 is a more potent KCNQ2/3 as shown in cell-based assay and is highly efficacious in a broad range of epilepsy and pain animal models. Many research data support ZM-003 to have a much more favorable pharmacokinetics and safety profile for a once daily dosing in human. ZM-003 is currently under IND-enabling studies. |
| Target | KCNQ2; KCNQ3 |
| Drug Modality | Small molecule |
| Indication | Epilepsy |
| Product Category | Voltage-Gated K(V) 7.2/7.3 (KCNQ2/3) Channel Activators |
| Mechanism of Action | ZM003 reduces nerve excitability by activating KCNQ2/3 potassium channel. |
| Status | IND |
| Patent | Granted |
Protheragen Inc. is actively seeking partnership for ZM-003. Potential collaboration can be strategic alliance, licensing, or marketing agreement.
We look forward to hearing from you.
The M channel is a slowly activating and deactivating potassium channel that plays a critical role in determining the subthreshold electrical excitability of neurons as well as the responsiveness to synaptic inputs. The M channel is formed by the association of the protein encoded by KCN2 and a related protein encoded by the KCNQ3 gene, both are integral membrane proteins. KCNQ2 and KCNQ3 are expressed in an overlapping distribution in brain, with high levels in critical areas for seizures, including the hippocampus, neocortex and thalamus, but are not expressed in most other tissues.
| Approved Name | Potassium voltage-gated channel subfamily Q member 2 |
| Official Symbol | KCNQ2 |
| Gene Type | Protein coding |
| Synonyms | EBN; BFNC; EBN1; ENB1; HNSPC; KV7.2; KCNA11 |
| Ensembl | ENSG00000075043 |
| Gene ID | 3785 |
| mRNA Refseq | NM_172109 |
| Protein Refseq | NP_742107 |
| OMIM | 602235 |
| UniProt ID | O43526 |
| Chromosome Location | 20q13.33 |
| Approved Name | Potassium voltage-gated channel subfamily Q member 3 |
| Official Symbol | KCNQ3 |
| Gene Type | Protein coding |
| Synonyms | EBN2; BFNC2; KV7.3 |
| Ensembl | ENSG00000184156 |
| Gene ID | 3786 |
| mRNA Refseq | NM_004519 |
| Protein Refseq | NP_004510 |
| OMIM | 602232 |
| UniProt ID | O43525 |
| Chromosome Location | 8q24.22 |
| Gene Function | Defects in KCNQ2 or KCNQ3 are the cause of benign familial neonatal convulsions. At least five transcript variants encoding five different isoforms have been found for KCNQ2. Alternative splicing of KCNQ3 results in multiple transcript variants. |
| Pathway | KCNQ3 associates with KCNQ2 to form a potassium channel to regulate neuronal excitability. |
| Major Conditions | Pain; Neurological Disorders; Musculoskeletal and Connective Tissue Disorders |
ZM-003 is a small-molecule KCNQ2/3 modulator modified from Retigabine (INN). Comparing with Retigabine, ZM-003 has better chemical and metabolic stability, antiepileptic activity, pharmacokinetic properties, and safety/tolerability profile. It does not form colored dimers like Retigabine, eliminating the side effect of forming skin and retinal pigmentation.
Epilepsy is a chronic neurological symptom complex that confers susceptibility to recurrent, unprovoked seizures caused by abnormal neuronal firing in the brain, affecting up to 70 million people worldwide. The psychological and social consequences of epilepsy are significant and may seriously affect the quality of life. As a hidden disability, patients are vulnerable to stigmatization and subsequent discrimination and, in many cases, violation of common rights. This is especially common in underdeveloped societies, where the disorder is more prevalent and at the same time, there is a poorer understanding of the disease.
According to the world health organization’s global burden of disease (GBD) 2015 study, epilepsy was the cause of death of 124,900 (range 119,300 to 131,000) people worldwide in 2015, yielding an age-standardized death rate of 1.7 (range 1.6 to 1.8) per 100,000.
Every human being has a brain seizure threshold, which makes them more or less resistant to seizures. Epileptic patients have an abnormally low seizure threshold that can be normalized through treatment. Most antiepileptic drugs increase the threshold of electrical energy in the epileptic focus required for a seizure to occur. Therapeutic strategies include potentiating the inhibitory pathways within the central nervous system, inhibiting excitatory glutamatergic pathways or inhibiting excessive neuronal firing.
The existence of a low-threshold, non-inactivating voltage-dependent potassium current in neurons, referred to as the M-current, plays a dominant role in regulating excitability because of its unique activity in the voltage range of action-potential initiation. The M-current slowly activates when an excitatory stimulus depolarizes the neuron toward spike threshold, repolarizing the membrane towards resting potential and suppressing firing. In this way, the M-current limits repetitive spike firing in response to a persistent depolarizing stimulus and is therefore, a key mechanism for “spike-frequency adaptation.” Suppression of the M-current results in membrane depolarization and an increase in neuronal input resistance, making the cell more likely to fire action potentials.
Because KCNQ2 and KCNQ3 constitute an M-current in native neurons, ZM-003 acts as an opener (activator) of the KCNQ2/KCNQ3 potassium channel by dramatically shifting the activation voltage to more negative potentials, and also speeding the rate of activation and slowing deactivation, which means that the M-current is enhanced to reduce excitability and counteract convulsions.
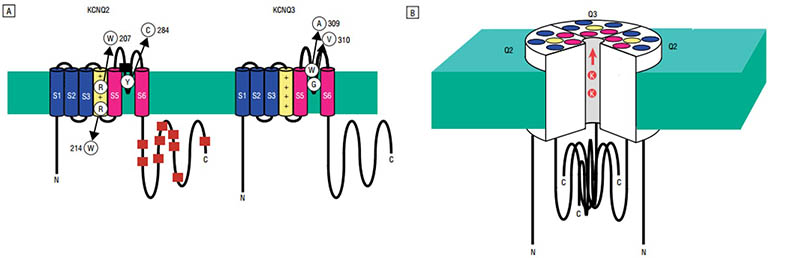
Figure. The structure of M-channels. (JAMA Neurology 60(4):496-500, May 2003)
ZM-003 is currently in IND-enabling studies. Part of the efficacy, therapeutic index, and safety studies in vivo have been completed.

Please feel free to contact us for more research data.